Abstract
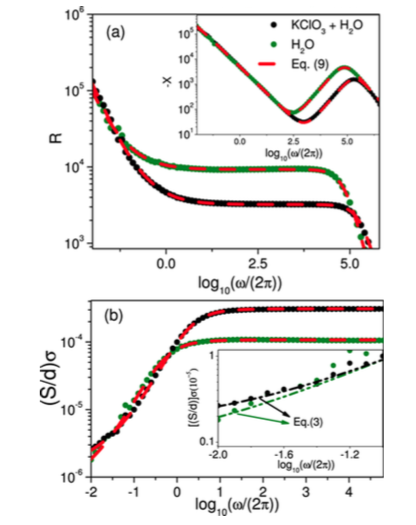
In this study, we argue that ion motion in electrolytic cells containing Milli-Q water, weak electrolytes, or liquid crystals may exhibit unusual diffusive regimes that deviate from the expected behavior, leading the system to present an anomalous diffusion. Our arguments lie on the investigation of the electrical conductivity and its relationship with the mean square displacement, which may be used to characterize the ionic motion. In our analysis, the Poisson−Nernst−Planck diffusional model is used with extended boundary conditions to simulate the charge transfer, accumulation, and/or adsorption−desorption at the electrode surfaces.
Type