Nearest neighbor permutation entropy detects phase transitions in complex high-pressure systems
Abstract
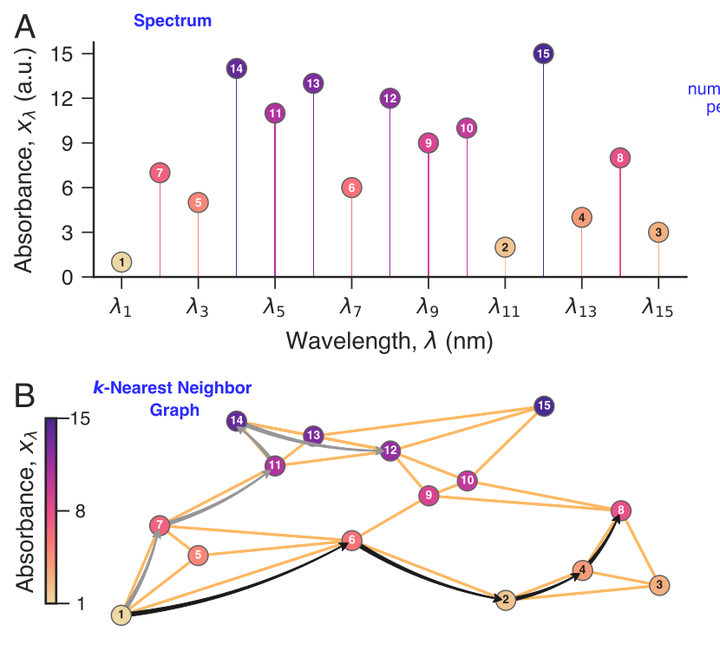
Understanding the high-pressure phase behavior of carbon dioxide-hydrocarbon mixtures is of considerable interest owing to their wide range of applications. Under certain conditions, these systems are not amenable to direct visual monitoring, and experimentalists often rely on spectrophotometric data to infer phase behavior. Consequently, developing computationally efficient and robust methods to leverage such data is crucial. Here, we combine nearest neighbor permutation entropy, computed directly from in situ near-infrared absorbance spectra acquired during depressurization trials of mixtures of carbon dioxide and a distilled petroleum fraction, with an anomaly detection approach to identify phase transitions. We show that changes in nearest neighbor entropy effectively signal transitions from initially homogeneous mixtures to two-phase equilibria, thereby enabling accurate out-of-sample online predictions of transition pressures. Our approach requires minimum data preprocessing, no specialized detection techniques or visual inspection of the spectra, and is sufficiently general to be adapted for studying phase behavior in other high-pressure systems monitored via spectrophotometry.